Premium Only Content
This video is only available to Rumble Premium subscribers. Subscribe to
enjoy exclusive content and ad-free viewing.
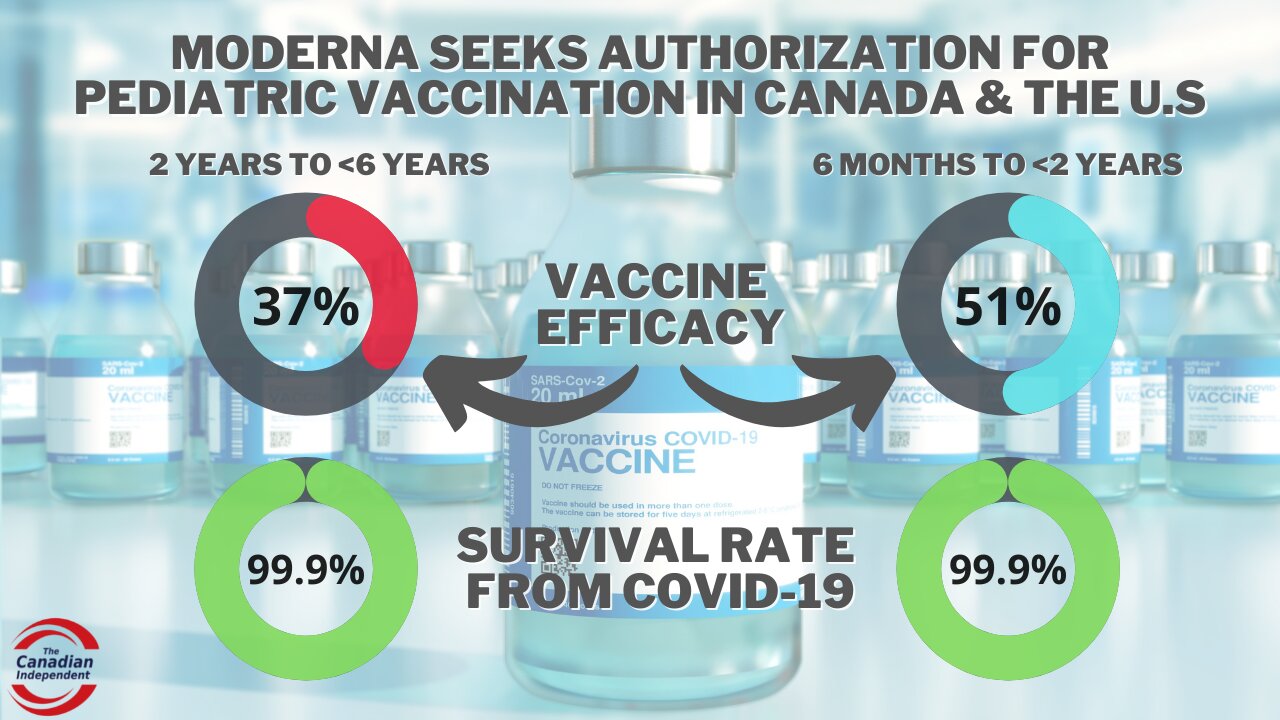
Moderna seeks EUA for kids under 6 years with high survival rate & low vaccine efficacy results
3 years ago
339
Moderna submits emergency use authorization application to Canada and the U.S. for their COVID-19 vaccine in children 6 months to under 6 years with a 37% to 51% vaccine efficacy rate after two doses, children have a 99.9% survival rate in this age group.
Sources,
https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html
Loading 4 comments...
-
 7:37
7:37
The Canadian Independent
4 months agoWATCH: A young mothers bones rot away - doctors link it to the COVID-19 vaccine
1.47K5 -
 3:38
3:38
WTMJMilwaukee
3 years agoModerna vaccine for kids under 5
31 -
 1:54
1:54
Newsy
3 years agoModerna Seeks To Be 1st With COVID Shot For Kids Under 5
28315 -
 0:30
0:30
New York Post
3 years agoModerna says its COVID vaccine safe for kids under 6
66027 -
 1:30
1:30
KERO
3 years agoModerna seeks emergency use authorization for COVID-19 vaccine for younger kids
18 -
 0:40
0:40
WPTV
3 years agoModerna asks F.D.A. to authorize its vaccine for children under 6
15 -
 1:31
1:31
KERO
3 years agoModerna seeks authorization for COVID vaccine for kids as young as six months
31 -
 0:52
0:52
Newsy
3 years agoModerna: Vaccine Produced Strong Immune Results In Kids
3525 -
 0:58
0:58
WFTS
3 years agoVaccine efficacy under question
8052 -
 1:17
1:17
Reuters
3 years agoModerna says COVID shot for kids under 6 is safe
1485